Properties Of Ionic And Covalent Compounds
Ch150 chapter 4 covalent bonds and molecular compounds chemistry Difference between covalent and ionic bonds chemistry education. Ppt combined ionic metallic and covalent compounds powerpointIdentifying ionic and covalent bonds worksheet answer key.
Properties Of Ionic And Covalent Compounds
Web Sep 20 2022 nbsp 0183 32 Physical Properties of Ionic Compounds Melting Points Shattering Conductivity Summary Review Explore More Figure PageIndex 1 In nature the ordered arrangement of ionic solids gives rise to beautiful crystals A Amethyst a form of quartz ce SiO 2 whose purple color comes from iron ions Ppt ionic metallic and covalent compounds powerpoint presentation. Lab ionic and covalent compounds solutionsHow do you determine if a substance is an ionic compound socratic.
CH150 Chapter 4 Covalent Bonds And Molecular Compounds Chemistry
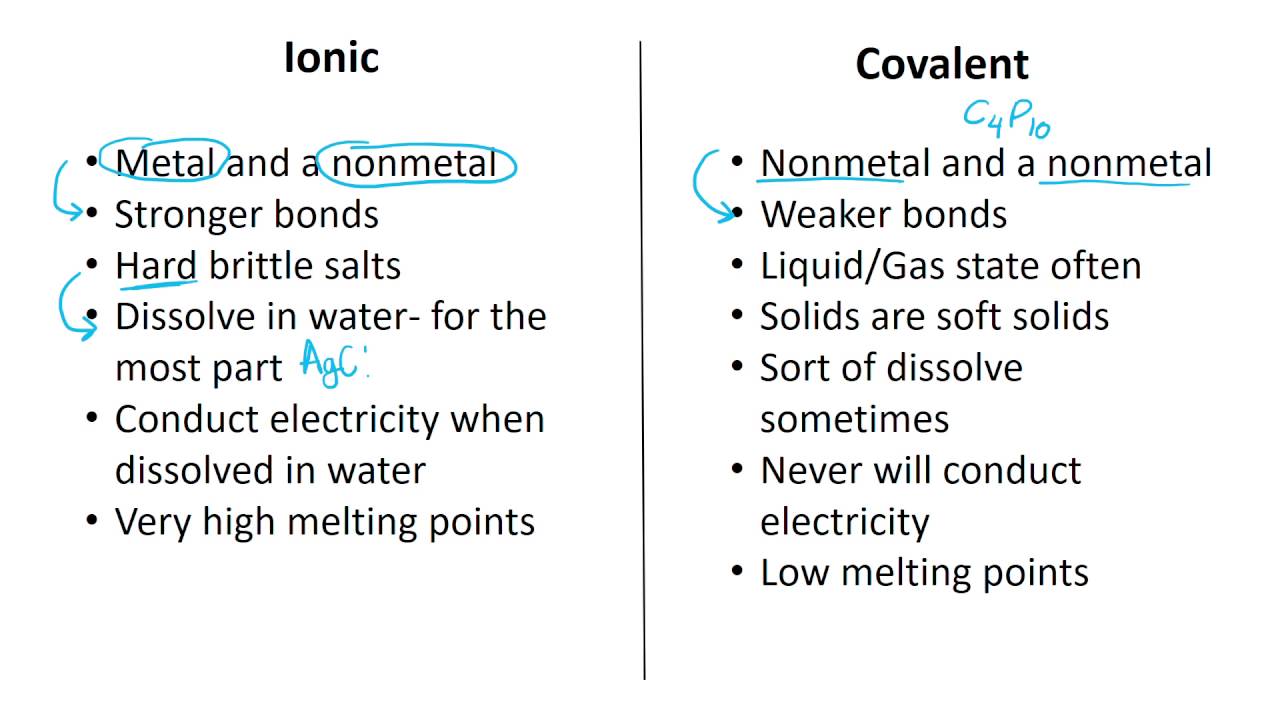
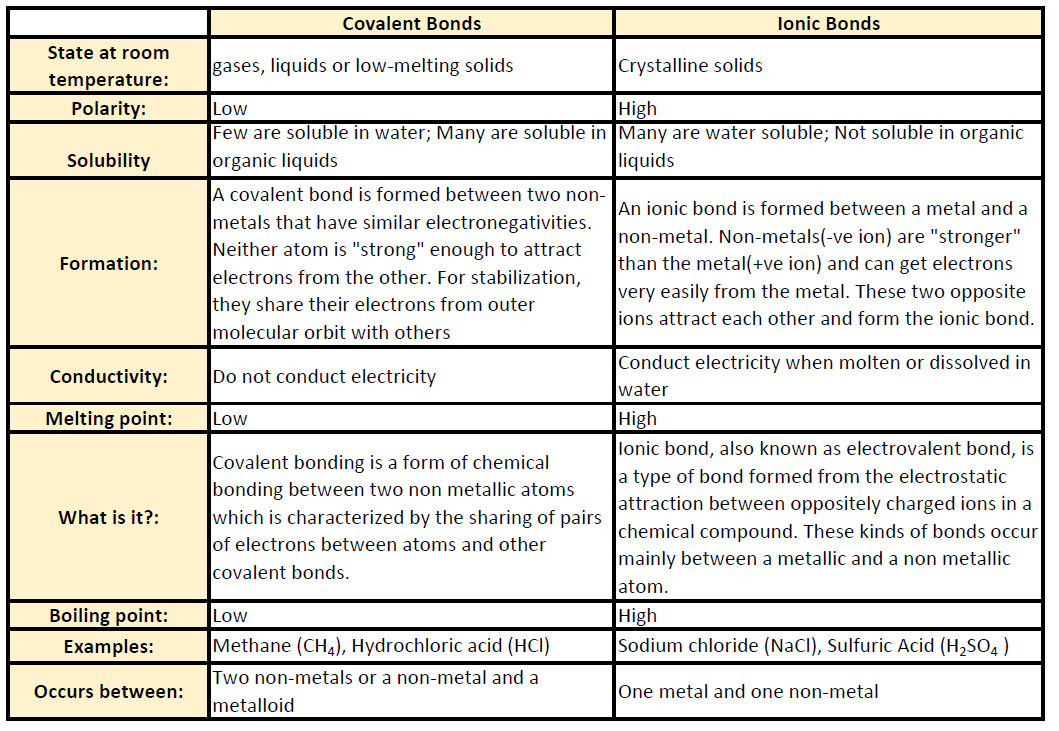
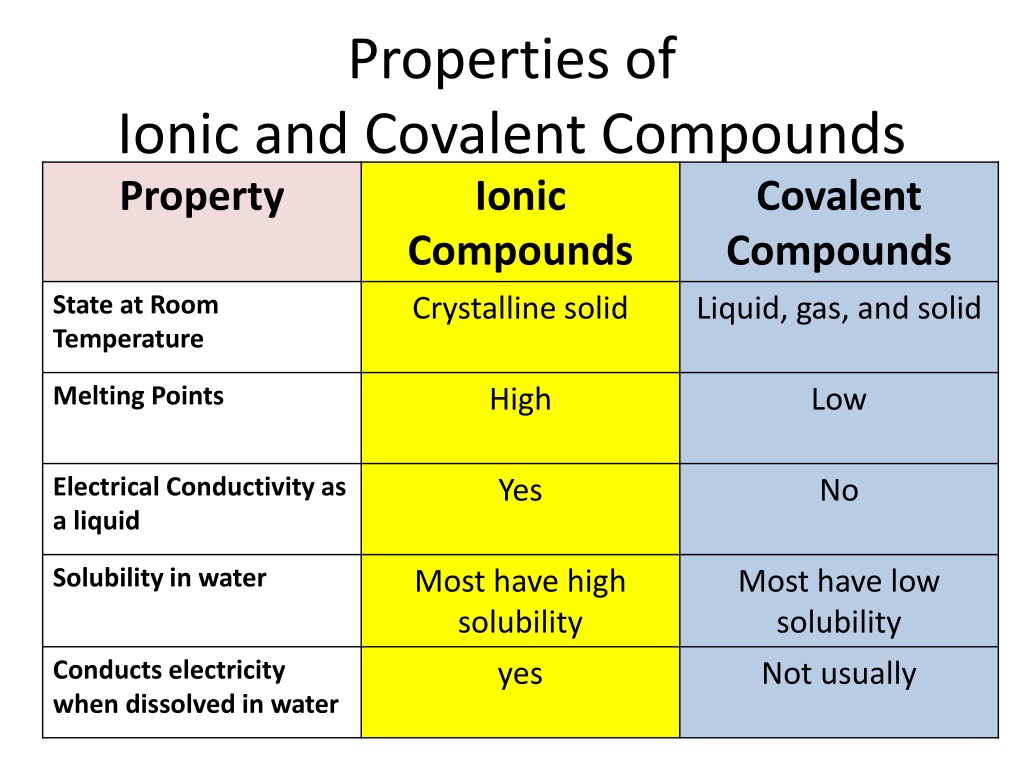
Web Ionic compounds generally form from metals and nonmetals Compounds that do not contain ions but instead consist of atoms bonded tightly together in molecules uncharged groups of atoms that behave as a single unit are called covalent compounds Covalent compounds usually form from two or more nonmetals ;The ions are held together by ionic bonds: electrostatic forces of attraction between oppositely charged cations and anions. The properties of ionic compounds shed some light on the nature of ionic bonds. Ionic solids exhibit a crystalline structure and tend to be rigid and brittle.
PPT 2 3 Classifying Chemical Compounds Properties Of Ionic And
Properties Of Ionic And Covalent CompoundsCompounds can be classified as ionic or covalent. Molecules are the simplest unit of a covalent compound, and molecules can be represented in many different ways. Atoms are the smallest units of matter that still retain the fundamental chemical properties of … Web What are the main properties of ionic compounds There are many properties Here is a short list of main properties They form crystals Ionic compounds form crystal lattices rather than amorphous solids They have higher enthalpies of fusion and vaporization than molecular compounds They are hard They are brittle